


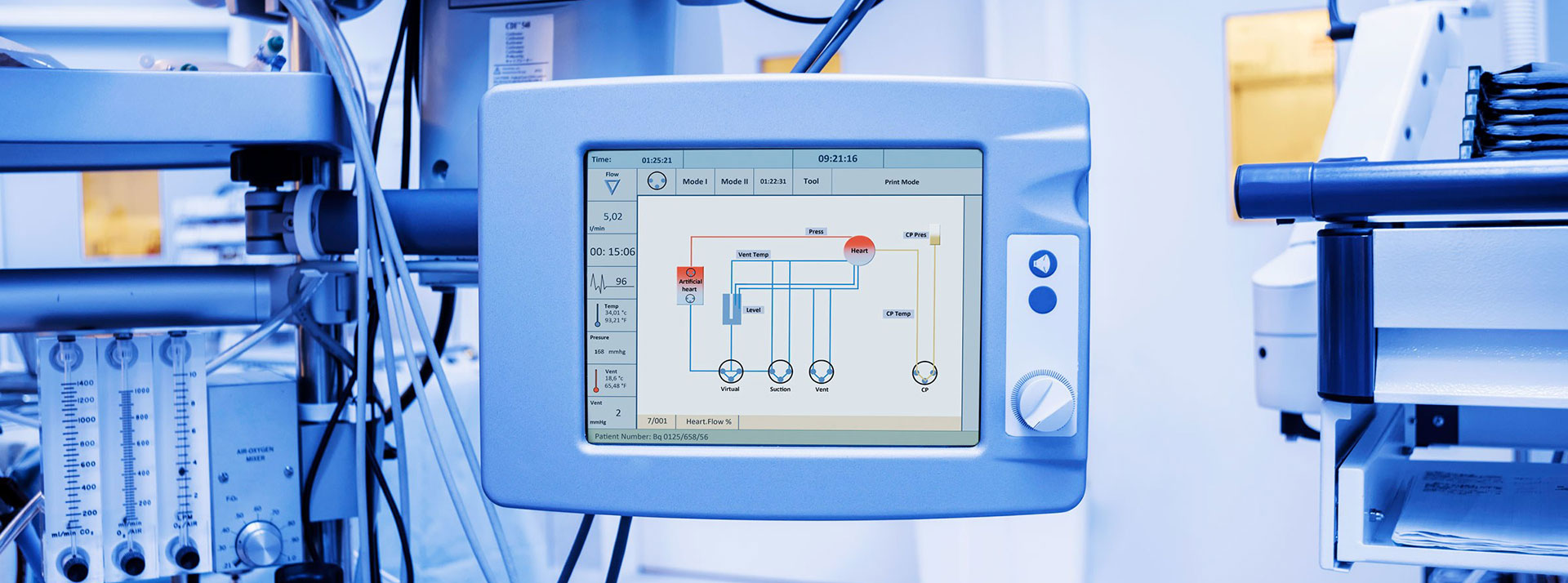
Apart from regular maintenance, medical devices should be tested regularly to ensure proper, safe and efficient operation. In the health sector, medical devices and equipment are produced in accordance with the relevant legal regulations. For example, the NFPA 99 standard issued by the National Fire Protection Association sets risk-based criteria in health facilities to minimize fire, explosion and electrical hazards.
Medical devices directly affect human life. In order to provide safe and effective health care services to patients, companies producing medical devices must follow validation practices as well as tests to ensure the quality and reliability of these devices. Medical devices and equipment are subject to numerous legal regulations. End users expect exceptional performance, efficiency and security from these devices.
The manufacturer must establish a strategy for testing medical devices and prepare compliance processes for better performance and effectiveness of these devices. For better testing coverage, each functionality of medical devices should be tested from the design stage. Otherwise, solving future problems requires more labor, time and cost.
An effective medical device testing strategy requires several testing requirements based on component characteristics, manufacturing process and other critical functional characteristics of the device. Test requirements define setup conditions, actions, and expected response constraints for each test. These requirements have different parameters at different stages of the manufacturing process, from component selection to final assembly of the medical device.
In order to test at the highest standards, medical devices must undergo rigorous electronic testing. Today, microprocessors are used in most medical devices. Therefore, medical device tests start with a microprocessor test. In addition, test automation systems are used in the tests. These systems consist of a computer and software used to control the testing process.
The application is received, the contract and then the product, vehicle and vehicles for which, what kind of service is determined exactly.
The necessary laboratory environment is prepared and the products, tools and instruments requested by the organization are tested by experts with the reference of the existing standards and measurements are made.
The data obtained as a result of meticulously completed tests, measurements and analyzes are evaluated and accredited approved reports are submitted by expert engineers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.